December 9th,2013
H18
Period 5
Technetium-99m
( pictured above) element from periodic table.
Background:
All isotopes in Technetium are radioactive. Technetium has many different isotopes, however, technetium-99m is the most abundant. It's symbol is Tc-99m and is a silver-gray, metal. The 'm' in Technetium-99m is stands for metastable. It has a half-life* of about 6 hours. This is considerably long for it to decay. Because it takes so much time for it to decay, the state of the this isotope is a metastable* state.
*Half-life: the amount of time in takes for the atoms in the isotope in decay into a stable form.
*Metastable: chemically unstable in the absence of certain conditions that would induce stability, but not liable to spontaneous transformation.
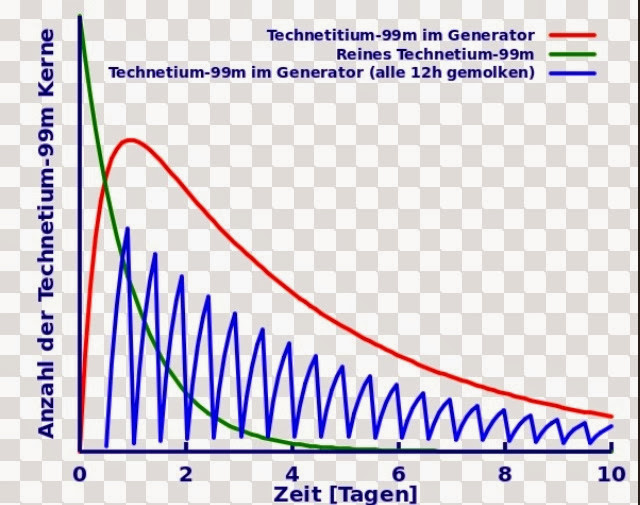
* (pictured above) decay of Technetium-99m
Modern-Day Usage:
Technetium-99m is a tool used in nuclear medicine. It is used in scans and helps doctors see internal organ such as the brain ( pictured above) and the lungs. Technetium-99m has gamma ray energy. It gives off radiation when in use, but so little that in does not affect the human body. Scans are used about 20 million times a year. An example of this would be Technetium Tc 99m Pentetate (Injection). This is used to see images of the kidneys and the brain to see if they are functioning properly. This injection is a radio pharmaceutical, radioactive agents used to find and treat certain diseases and illnesses and/or to study the organs' function.
(pictured above) an example of a brain scan which shows the differences between the brain when it is stressed and the brain when it is rested.
Other:
I believe that Technetium-99m has been very useful in the medical field today. Because of the scans that can be used on humans and other animals, doctors and scientists have been able to study the condition of certain organs under certain conditions.
Sources:
www.epa.gov
www.faculty.virgina.edu
www.ie.lbl.gov ( Ms. Janik's site)
www.cyberphysics.co.uk
www.dictionary.com
www. ncbi.hlm.nih.gov



No comments:
Post a Comment